Biomedical Science and Research Journals | The Structures and Functions of Vaccinia Virus E3L and Protein Kinase R
The Structures and Functions of Vaccinia Virus E3L and Protein Kinase R
Abstract
The Vaccinia Virus (VV) E3L of the poxvirus family encodes double-stranded RNA-binding, which promote viral growth and pathogenesis by inhibiting innate immunity. E3L gene of vaccinia virus also acts as a multifunctional viral immune factor that blocks host defense factors from participating in the induction and action of Interferon (IFN). In other words, the main function of E3L is to block the activations of Interferon Regulatory Factor 3 (IRF3), RNase L, and Protein Kinase R (PKR) simultaneously. These inhibitions may be achieved by combining Double-Stranded RNA (dsRNA) isolation with direct inhibitions of protein-protein interactions. Meanwhile, dsRNA-dependent serine/threonine protein kinase induced by IFN plays a key role in innate immune response to viral infection and key regulatory roles during signal transduction, cell proliferation and differentiation, and apoptosis. Furthermore, activation of PKR inhibits protein translation by Eukaryotic Translation Initiation Factor 2-Alpha Kinase 2 (EIF2AK2) at peak viral infection. In this review manuscript we focus on the activity of the vaccinia virus E3L protein and in the PKR host protein, describing their functions and interactions to provide new ideas regarding for vaccine development and the developments of antiviral drugs.
Keywords: E3L; Double-stranded RNA; Protein Kinase R; Interferon, Orthopoxviruses, Immune, Biological Functions, Poxviridae, Vaccinia Virus E3L Protein, Polypeptide, Enzyme
Introduction
Poxviridae belongs to the biological diversity family of a large dsDNA virus, which is characterized by replication in the cytoplasm of infected cells [1]. Poxviruses encode many immunomodulatory proteins, and the Orthopoxviruses encode many genes, such as E3L, C3L, and K3L, which exhibit various biological functions [2]. Among them, E3L is the most widely studied vaccinia gene due to the best biological characteristics of the vaccinia virus E3 protein, such as host range function and inhibition of innate immune response, apoptosis and antiviral activity induced by interferon, and it is expressed early in the viral replication cycle and known to inhibit multiple innate immune pathways [3]. In addition, E3L has been successfully used as a smallpox vaccine in several countries and constitutes an important bioterrorism threat, and thus, it is an important current research topic. Previous studies have shown that vaccinia virus gene products E3L interfere with the functional activity of double-stranded RNA-dependent Protein Kinase (PKR) in the IFN system [4]. The vitro analysis of Orthopoxviruses showed that the inhibitory effect of E3L gene products on PKR was 50-100 times higher than that of K3L gene products. Whereas the E3L inhibitor can bind with the double-stranded RNA of protein kinase but the K3L inhibitor did not. The mechanism of action of these two inhibitors is different, but the molecular mechanism is not yet revealed.
Biological Function of Vaccinia Virus E3L
E3L protein is highly conserved in Poxviridae and related viruses and plays a key role in eluding host cell Interferon (IFN)- mediated defense, for example, The sequence alignment (Figure 1A & 1B) of the exhibits sequence similarities with C3L (variola virus) (93.68%), HSPV060 (horsepox virus) (97.89%), CPXV069 (cowpox virus) (93.7%), and RPXV048 (rabbitpox virus) (97.4%) shows that The E3L gene is conserved among a large fraction of the members of the Poxviridae. The gene’s protein product, E3, is a doublestranded RNA binding protein. It can affect host range and is used by orthopoxviruses against cellular defense pathways such as PKR and RNase L [5]. Vaccinia virus encodes a secretory polypeptide structurally associated with complement control proteins. The E3L protein protects the virus from the immune system complement attack by inhibiting the classical and alternative complement activation pathway response process.
The main function of the vaccinia virus gene E3L is to encode an intracellular protein that affects the innate immune response. Analysis of the coding of the vaccinia virus genome shows that there are about 200 Open Reading Frames (ORFs) [6] with the non-essential genes located in the variable terminal regions and the essential genes are mainly located in the highly conserved central region of the genome [7, 8]. Three genes in the vaccinia virus genome encode the BTB/kelch proteins: C3L, F3L and A55R. These proteins collectively contain a C-terminal kelch domain and an N-terminal BTB (broad-complex, tram-track and bric-abrac) domain. The kelch motif sequence is 44-56 amino acids long and occurring as four to seven repeats [9]. The E3L has the biochemical ability to bind Z-form DNA to N-terminal and dsRNA to C-terminal dsRNA Binding Domain (DRBM) and its typical biological functions include cytokine and IFN-induced antiviral activity inhibition (Figure 1A). However, it has not been established how dsRNA binding by E3L influences its biological function.
The E3L can inhibit the innate response to double-stranded RNA and block the internal apoptotic pathway, so there is a regulatory link between vaccinia virus proteins and double-stranded RNA [10,11]. Type I Interferon (IFN) has a prominent role in innate and adaptive immunities by enhancing the function of dendritic cells; it can induce monocyte differentiation, promote the transformation of immunoglobulin in B cells, and stimulate the effector function of T cells. Increased production of IFN/plasma dendritic cells not only has an efficient role in anti-virus defense but also may be a pathological factor of many autoimmune diseases. The IFN resistance gene E3L contains an N-terminal domain is required for virulence, which mediates interactions with Z-DNA, PKR interaction, and nuclear localization. Furthermore, the C-terminal domain of E3L is required for IFN resistance and E3L host range: it binds with double-stranded RNA to inhibit IFN-induced PKR activation. Meanwhile, the N-terminal Z-DNA binding motif also exists in adenosine deaminase of cellular dsRNA-binding protein.
Biological Function of Protein Kinase R
Double-stranded RNA-dependent Protein Kinase R (PKR) is a serine-threonine kinase gene sequence containing 551 amino acids, and it is functionally regulated by the EIF2AK2 gene (located on chromosome 2) in human. PKR is important in mRNA translation, the regulations of transcription, apoptosis, proliferation, and other important cell processes [12]. Moreover, disruption of PKR homeostasis is believed to be associated with cancer, neurodegeneration, inflammation, and metabolic disorders, and as such has attracted research attention [13]. PKR is widely expressed in vertebrates but not in plants, fungi, protozoa, or invertebrates. It was first cloned at the Pasteur institute in 1990 and is also known as interferon-induced double-stranded RNA domain kinase.
The N-terminal of PKR is connected to a flexible C-terminal kinase domain. DRBMs are required for high-affinity interactions with dsRNA (Figure 1A) [14], and the catalytic domain of PKR is in the region involved in dimerization with a typical kinase. PKR maintains the stability of the cellular homeostasis, which is critical in the process of cell infection. It is an enzyme with an interferon-inducible effect and activated by a mechanism that involves double-stranded RNA binding with its N-terminal region in an RNA sequence-independent fashion, which separates it from other kinases. However, the catalytic domain structure of PKR is like those of other protein kinases [15,16] (Figure 1C). EIF2AK2 interacts strongly with the C-terminal surface of PKR in a specific alpha-helix PKR-specific (G) kinase domain [17].
PKR is the central hub for detecting cellular stress signals and responses, so it is expected to be regulated through different stress response pathways. According to this concept, the typical activator of PKR is dsRNA, making PKR a pattern recognition receptor capable of regulating cellular function [18]. PKR’s central role in mediating the antiviral response is reflected in the highly positive selection as shown in the coding sequence and it suggests an arms race between PKR and the pathogens. However, PKR can be activated by other factors, such as heat shock proteins, heparin, and growth factors, meanwhile it is also activated in response to numerous insults, including nutrients or energy excess, cytokine calcium, non-viral pathogens, irradiation (probably by inducing DNA damage), and reactive oxygen species [19].
E3L Blocks the Activation of PKR
The dsRNA produced during virus replication is an important pathogenic molecular pattern that induces an antiviral immune response. In the virus infection detection process, the initial detection is completed by the innate immune; thus, the identify of virus dsRNA is an important early function. The response to dsRNA is mediated by several protein receptors that recognize the PAMP [20,21]. The most important of these mediating protein receptors are the two Caspase-Recruitment Domains (CARD)-containing helicase, a Retinoic Acid-Induced Gene I (RIG-I) and the related IFN-Induced Helicase I (IFN-I), as well as PKR [22]. One of the strategies that many viruses use to combat the antiviral immune response is to produce dsRNA-binding proteins. A commonly accepted model for the biological functioning of these viral dsRNA binding proteins involves binding to and isolation from viral dsRNA Pathogen-Associated Molecular Patterns (PAMPs) and inhibition of the associated antiviral immune response.
The IFN-induced PKR plays a key role in the innate immune response to viral infection, and this kinase also has an important role in the general processes of cell proliferation, cell apoptosis, signal transduction, and differentiation regulation [23]. It can also inhibit virus replication via phosphorylation of the alpha subunit of Eukaryotic Initiation Factor 2 (EIF2S1), effectively killing the virus. The phosphorylation process disrupts the EIF2S1 cycle between successive rounds of initiation, leading to translation inhibition and, ultimately, to the cessation of cellular and viral protein synthesis. In normal cells, PKR affects cell apoptosis and inhibits tumor phenotypes in response to various intracellular and extracellular signals. Since most tumor cells are resistant to certain apoptotic signals, we speculate that early molecular events in carcinogenesis and leukemia may include the inactivation of PKR expression or the activation of intracellular PKR inhibitors [24].
As can be seen in Figure 2A, E3L is a multifunctional viral immune factor that can block host defense factors from jointly participating in the induction and activities of IFN. The main role of E3L is to block the activation of PKR. This inhibition may directly inhibit protein-protein binding interactions through dsRNA isolation. Previous studies have shown that the E3L protein inhibits PKR by isolating the kinase's dsRNA activator, binding directly to PKR and inhibiting protein kinase activation [25]. A conclusion can be drawn that as a multifunctional pathogenic factor, E3L enables VV to evade the IFN system at multiple levels [26]. In HeLa cells, K3L gene product cannot competitively inhibit the high levels of PKR therefore vaccinia virus replication relies on the E3L gene product to isolate the low levels of dsRNA synthesized during virus replication. In BHK cells, E3L gene product cannot isolate the high levels of dsRNA synthesized, but K3L gene products can competitively inhibit PKR expression at lower levels without IFN treatment. The roles of PKR as a signaling molecule and the cellular responses induced by PKR through regulation of cellular signaling pathways have not been fully elucidated and further research is needed on the structural characteristics and functional mechanisms of these proteins [27].
The Structures of E3L and PKR
The ZBD structure of E3L is composed of three α-helices and three β-strands, and the folding mode is in α1β1α2α3β2β3 linear order (Figure 2B) [28]. Helices α1 and α3 are packed against a short anti-parallel, triple-stranded β-sheet, in which β3 is sandwiched between β1 and β2. Strands β1 and β3 are connected by two backbone H-bonds while strands β2 and β3 are bridged by H-bonds. Loop 2 between α2 and α3 is less rigid whereas all other loops are tightly structured. Loop 4 between β2 and β3 is rigid because of the two serial prolines, of the former uses a rare cis-peptide bond. In the Z-DNA co-crystal structure, the protein makes many important Van der Waals interactions with Z-DNA [29]. The structure of vaccinia virus E3L contains a conserved DRBM domain at the C-terminal half sequence, which is need in vitro dsRNA binding; moreover, this region provides host coverage and has a key role in replication efficiency and IFN resistance [30]. The C-terminal portion of the DRBM-containing E3L exists as a dimer in solution and seems to synergistically bind dsRNA.
Therefore, protein-protein interaction can contribute to the binding affinity between E3L and dsRNA. The early expression of E3L gene in the viral replication cycle inhibits a variety of innate immune system responses. In terms of protein structure and function, E3L has the biochemical ability to bind Z-form DNA to N-terminal and C-terminal DRBM [31]. The structure of PKR includes an N-terminal dsRNA in which two tandem repeats of conserved double-stranded RNA binding motifs (DRBM1 and DRBM2) are separated by 23 amino acids followed by a flexible ligand connected to the C-terminal kinase domain. The DRBMs are required for the high-affinity interactions with dsRNA. The catalytic domain of PKR is in the region where dimerization occurs with a typical protein kinase that produces a pleated β-sheet N-terminal lobe and an alpha-helical C-terminal lobe. The PKR kinase domains form alternating Back-To-Back (BTB) and Front-To-Front (FTF) contacts produces an extended, filament-like assembly. The activation reaction is produced by the binding of PKR to viral RNA, the autophosphorylation is induced on conserved threonine residues located in the active segment of the kinase domain.
Mutants of E3L DRBM
In most cell-based experiments, vaccinia virus is observed to be significantly resistant to the antiviral effect of IFN and can rescue IFN-sensitive viruses, such as vesicular stomatitis virus and encephalomyocarditis virus, from the antiviral effects of IFN [31]. During the evolution of viruses, many viruses have developed mechanisms to prevent antiviral establishment by inhibiting components of PKR pathway. In the IFN system, vaccinia virus E3L needs to develop resistance to the IFN system. The experimental results of the dsRNA binding, virus rescue, and PKR inhibition about the E3L virus mutants have shown in Figure 2C [26]. Several E3L mutants bind to dsRNA and inhibit PKR in vitro.
Deletion of 26 C-terminal amino acids from the E3L-encoded protein destroyed the ability of the E3L protein to bind to dsRNA and inhibit PKR; moreover, it did not rescue replication of vaccinia virus to a greater extent than that of the parental plasmid. Mutations of E3L G164V and A174V also destroyed the ability of the E3L-encoded protein to bind dsRNA and inhibit PKR, respectively. However, deletion of 83 N-terminal amino acids from the E3L-encoded protein did not affect dsRNA binding or PKR inhibition. In addition, mutations of G164A or 174G did not appreciably affect dsRNA binding or PKR inhibition and both G164A and A174G rescued vaccinia virus replication. The rescued replication of vaccinia virus was less efficient than that from wild-type E3L. Finally, deletion of seven C-terminal amino acids from E3L reduced the affinity of the encoded proteins for dsRNA. For each of the mutants analyzed, E3L-encoded proteins were expressed in HeLa cells at comparable levels, suggesting that differences in the efficiency of vaccinia virus rescue were not due to differences in the levels of expression [32].
Mutants of PKR
PKR has the characteristics of antivirus, antiproliferation, anti-tumor properties, and exists as a translation inhibitor in the IFN system. The dsRNA binding domain was in the N-terminal region of PKR, and it has two nearly identical regions with similarly effect: DRBM1 and DRBM2. However, in mammalian cells, dsRNA-binding mutants have a partial transposing dominance over wild type PKR, suggesting that dsRNA-activator binding is not the mechanism of PKR mutant phenotypes [33]. The DRBM1 region plays a decisive role in the dsRNA binding of PKR, while DRBM2 is not absolutely required of the process. Several studies have used bacteria, yeast, and mammalian cells to measure the functions of various mutants and confirm the importance of these residues for PKR function. As can be seen from the Table 1, the dsRNA binding of the two mutants (G57A and K60A) was completely lost, while the binding function of the other two mutants (K64A and L75A) remained but the efficiency was less than 10% of the wild type. Lysine’s at 60 and 64 sites are important residues for dsRNA binding (not 61 or 69), and it indicates that different lysine residues do not have the same binding effect [33].
In vivo and vitro, the ability to bind dsRNA is directly related to the function of PKR, and dsRNA binding is not the mechanism by which mutant PKR is superior to wild type PKR. In another study of mutations in the DRBM region: the DRBM1 region was deleted from the wild type PKR, after adding this DRBM1 lacking mutation into NIH3T3 cells, found that it is resulting in malignant transformation and tumor development in nude mice. PKR variants completely lacking DRBM1 were largely unresponsive to dsRNA inactivation assays but could be activated by heparin [34]. When catalytically inactive mutants are overexpressed in NHI3T3 cells, PKR regulates cell growth and acts as carcinogenic gene. Therefore, PKR as a tumor suppressor gene may have something to do with its translation-inhibiting properties and behaviors as a signal transducer in the proinflammatory reaction of unlike agents [35].
Conclusion Remarks
For the most typical biological functions of vaccinia virus E3L, such as the host range function and capacity for innate immune response inhibition, are mainly mediated by the DRBM region of the E3L, it is generally considered that the function of E3L is achieved by isolating the dsRNA produced by the virus, resulting in inhibition of the relevant cellular antiviral response. Since the E3 protein was first identified as having the biochemical capacity to bind dsRNA more than 20 years ago, some key questions about the molecular mechanisms by which the E3 protein functions remain to be resolved, so far, there are no direct experimental data showing that E3L binds dsRNA in virus-infected cells during the innate immune response process. But it has been demonstrated that the biochemical capacity of dsRNA binding is not necessary for the biological function of vaccinia E3. It is therefore important to understand how the biochemical binding capacity of dsRNA plays a role in the biological function of E3L [36].
Double-stranded RNA-activated Protein Kinase R (PKR) is a key regulator of the innate immune response and activation of PKR inhibits protein translation by the EIF2AK2 during the peak of viral infection. PKR also has a wide range of regulatory functions and some domains structures of PKR have been identified, but the overall structure still needs to be explored. In normal cells, PKR induces apoptosis and inhibits tumor phenotypes in response to various intracellular and extracellular signals. Since most tumor cells are resistant to certain apoptotic signals, it is highly likely that the early molecular processes in carcinogenesis and leukemia were the result of the inactivation of PKR expression, the activation of intracellular PKR inhibitors, or an indirect effect. E3L has a critical role in the pathogenesis of vaccinia virus by blocking the IFN system at multiple levels. The vaccinia virus E3L protein acts as a virulence factor due to its affinity to dsRNA and ability to inhibit PKR activity. In the absence of E3L, the IFN can effectively trigger PKR functioning. In summary, E3L can inhibit the IFN induction, and PKR is one of its main targets.
Previous studies have reported that vaccinia virus E3L binds to PKR and inhibits PKR activation in yeast systems and that dsRNA binding- defective E3L mutants can lose their PKR binding activity. However, as one of the research hotspots of immunomodulation proteins of vaccinia virus, we have integrated some key information related to this field in the hope that the field of pox virology/virology will benefit from this review. This study provides a novel suggestion for the development of antiviral drugs in humans and suggests a theoretical basis for the further development of vaccines and the manufacture of novel antiviral compounds.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2018R1D1A1B07043701) to S.B.J and (2016R1D1A1B02011142) to M.S.J.
For More information: https://biomedgrid.com/fulltext/volume10/pathophysiological-aspects-of-the-use-of-hypobaric-hypoxic-training-in-the-treatment-of-anemia-in-chronic-glomerulonephritis.001527.php
Know More Information: Biomed Grid Please Click on : https://biomedgrid.com
For More information: Open Access Journals of Biomedical Science and Research: Journal of Biomedical Science
Know More Information: Biomed Grid Please Click on : https://biomedgrid.com
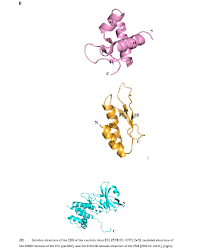
Comments
Post a Comment