Biomedical Science and Research Journals | Arab Society for Pediatric Endocrinology and Diabetes (ASPED) Masterclass in Pediatric Bone Disease 2nd November 2018, Dubai, United Arab Emirates
Arab Society for Pediatric Endocrinology and Diabetes (ASPED) Masterclass in Pediatric Bone Disease 2nd November 2018, Dubai, United Arab Emirates
Abstract
The Arab society of Pediatric Endocrinology and diabetes
held a masterclass for pediatric bone diseases in Dubai, United Arab
Emirates (UAE) in
collaboration of Kyowa Kirin. This 1-day event was run within the
framework of the ASPED School and was attended by 76 delegates and 14
faculty.
Plenary lectures and. patient case reports were presented. The talks
covered all aspects related to rickets and highlighted that nutritional
vitamin D
deficiencies are relatively common. This is the case despite the
availability of proper nutrition and the adequate level of sun light. It
was emphasized
that is it vital to establish a diagnosis considering potential
underlying genetic abnormalities or comorbidities which may mask initial
laboratory
assessments and potentially lead to an unsuitable treatment choice.
Considering a less common genetic form of rickets is important in the
region
owing to the high frequency of consanguinity. It has been pointed out
that even when a correct diagnosis and initial management is selected,
it is
essential to follow up patients regularly and adjust their treatment to
match their needs during different growth phases.
Overall, the 14 regional and international speakers discussed
nutritional and hereditary rickets, disorders of phosphate homeostasis,
management of hypophosphataemic rickets and skeletal disorders beyond
rickets. Lively discussions around all presented topics dominated the
day, with an interest in new treatments, including burosumab as a novel
fully human anti-FGF23 monoclonal antibody therapy. Burosumab has
demonstrated effective inhibition of the FGF23-mediated pathway and has
received approval by the US Food and Drug Administration (FDA) for
the treatment of adults and children with X-linked hypophosphataemia
(XLH). Preliminary experience in the region of its use has been
presented.
The Arab Society for Pediatric Endocrinology and Diabetes (ASPED) www.asped.orgmmary
ASPED was launched in Abu Dhabi, UAE in September 2012, upon the initiative of a group of pediatric endocrinologists from the Middle East and North Africa. The society is a non-profit scientific organization and is registered under the Dubai Association Center (DAC) under license number DAC-0001. Its aim is to ensure a high standard of care and development in the field of pediatric endocrinology and diabetes in the Arab region extending from the Gulf through the Northern African countries.Kyowa Kirin
Founded in 1949 in Japan, Kyowa Hakko Kirin Co., Ltd (KHK) has a track record in Japan and is now expanding globally. Its daughter company, Kyowa Kirin International (KKI) showed a rapid growth in pharmaceutical industry in various therapeutic areas including oncology, nephrology and central nervous system disorder. Many of KKI’s therapies are based on antibody technology with enhanced antibody efficacy and safety.Acknowledgement
ASPED is grateful to all the speakers and moderators (regional and international) who contributed to the course and made it a meeting appreciated for its high educational level. We also thank Kyowa Kirin, Gulf for its collaboration and support of pediatric bone disease education in our region.Kyowa Kirin-ASPED Pediatric Bone Disease
Masterclass
The Kyowa Kirin-ASPED masterclass in pediatric bone disease was a 1-day meeting as part of a 3-day ASPED School at the Holiday Inn, Festival City, Dubai, 31 October-3 November 2018. The masterclass aimed to educate, empower and update physicians practicing in Arab countries and involved in the care of young people with bone disorders. The masterclass was planned to be a platform to share expertise and the latest developments in clinical approaches to treatment of diseases linked to metabolic and genetic bone diseases. Including prominent international and regional speakers, the masterclass attracted 76 attendees from the Middle East and African countries.Presentation Summaries
Nutritional and Hereditary Rickets
Aetiology and Treatment of Rickets: an overviewAbdelhadi Habeb, Kingdom of Saudi Arabia (KSA): Maternity and Children Hospital & Prince Mohammed bin Abdulaziz Hospital, KSA. Rickets occurs relatively commonly in the Middle East, Africa, and Asia. It is a gender-independent condition that starts typically between the ages of 3 and 18 months resulting in weak or soft bones in children. Although first described in 1650, the treatment of rickets remained a medical challenge well into the 20th century Early treatments were based on cod liver oil trials, and experimentation with sunlight exposure. Four subtypes of rickets are commonly distinguished and include vitamin D-related, hypocalcemia-related, hypophosphatemia-related and secondary rickets with alternative causes, such as cancer.
Hypophosphatasemia is fundamental in the development of rickets, and it was recognized early on that vitamin D deficiency, hereditary or nutritional, is the leading cause of reduced intestinal calcium absorption, resulting in increased renal phosphate excretion via activation of the parathyroid hormone pathway. Hypophosphatasemia results in accumulation or impaired apoptosis of the hypertrophic chondrocytes in the growth plate of long bone joints without enough calcification; this leads to symptoms such as bowed legs, stunted growth, bone pain and swollen joint areas. Complications may include bone fractures, muscle spasms, an abnormally curved spine or intellectual disability. Diagnosis is generally based on X-rays together with blood test findings of low calcium, low phosphorus, and high alkaline phosphatase (ALP) levels. Radiological assessment has been reported as not very successful in the diagnosis.
Treatment depends on the underlying cause or subtype of rickets. Enough vitamin D levels can be achieved through dietary supplementation and/or exposure to sunlight. Various vitamin D supplements are available, with vitamin D3 being a preferred treatment option as it is more readily absorbed than vitamin D2. However, vitamin D treatment for other forms of rickets may require calcium or phosphate supplementation to ensure appropriate metabolism of vitamin D.
Nutritional Rickets Revisited: Zulf Mughal, UK: Royal Manchester Children’s Hospital Manchester, UK. Nutritional rickets is a preventable disease and a result of insufficient vitamin D intake or absorption. A diagnosis of nutritional rickets is unlikely if there is a strong family history of rickets, associated features such as alopecia or hepatomegaly are displayed, severe skeletal deformities at a very young age or poor response to vitamin D therapy. In young children, manifestations of vitamin D deficiency include wide gait, swollen long bone joints, rachitic rosary, dental problems and osteomalacia in adolescents. In rare instances, cases of dilated cardiomyopathy have been reported.
While in early vitamin D deficiency, calcium levels are still normal and phosphate levels are decreased, patients with severe vitamin D deficiency display reductions in both calcium and phosphate. In rare cases of parathyroid hormone resistance, where calcium levels are reduced, and phosphate levels increased, calcium supplementation was seen to rescue the biochemical pattern of this phenotype. Calcium supplementation has shown to reverse symptoms of rickets in terms of radiological, histological and biochemical features. For infants 0-6 and 6-12 months of age, adequate calcium intake is 200 and 260 mg/day, respectively.
For children over 12 months of age, dietary calcium intake of <300 mg/day increases the risk of rickets independent of serum vitamin D levels. For children over 12 months of age, dietary calcium intake was classified as enough (>500 mg/day), insufficient (300- 500 mg/day) and deficient (<300 mg/day). Therefore, guidelines recommend treatment doses for 12 weeks based on age and level of deficiency with a follow-up maintenance dose as regular reassessment of the patient’s mineral status. In prevention studies, a dose of 400 IU daily has been shown as adequate to prevent rickets.
Rickets Case Reports
Case 1: Asmahan T Abdalla, Sudan: Gaafar Ibn Auf Pediatric Tertiary Hospital, Sudan Medical Specializations Board, Sudan. A case of rickets was reported in a female Sudanese child of consanguineous parents, despite high exposure to sunlight. The patient presented with recurring rickets on treatment discontinuation from the ages of 18 months to 20 years with clinical, laboratory and radiological features. First symptoms at 18 months old included floppy infant syndrome, open anterior fontanel, broad wrists, bowing of the legs and rosaries without organomegaly, skin, nail or hair abnormalities.
Following 6 months of conventional therapy of vitamin D3 and calcium, her walking abilities and laboratory parameters started to improve. Over the years she started having dental abnormalities and bone pain coupled with severe genu varum deformity. Repeat therapy alleviated the symptoms. The patient’s sisters presented at the ages of 2 and 12 years with similar symptoms, most prominently a very low vitamin D level at <7.5 ng/mL. Therefore, treatment with various doses of calcium and calcitriol was initiated and genetic testing was performed after investigations ruled out liver or renal disease and malabsorption.
Molecular analysis identified a previously unknown mutation (homozygosity at position 85 [C>T]), which resulted in a truncated and non-functional CYP2R1 gene. As a result of this analysis, 25-hydroxylase deficiency and, ultimately, vitamin D-dependent rickets type 1B was diagnosed. In general, even if genetic testing is not available, genetic rickets should be suspected in an environment with high levels of sunlight in a child who presents with history of rickets that is dependent on therapy or resistant to it, with history of consanguinity and a similar history in the family.
Case 2: Abdulla Al-Harbi, KSA: Madinah Maternity and Children Hospital, Madinah, KSA. A case of rickets was reported in the second child of consanguineous parents at 3 months of age following a fullterm pregnancy. The couple’s first child died at 3 months showing signs of hypocalcemia and skeletal deformities. The patient was bottle fed and received vitamin D3 supplement. However, supplementation was ineffective and lower limb deformities, wrist widening, and frontal bossing were observed at 14 months of age. Laboratory analyses demonstrated highly elevated ALP and parathyroid hormone (PTH) levels. Following a 1-week vitamin D3 washout, biochemical analysis was repeated showing even more increased ALP and PTH levels and calcium and phosphate levels below the normal range, while both 25-hydroxyvitamin D (25[OH] D) and 1,25-dihydroxycholecalciferol (1,25 [OH]2D) were normal.
To ascertain the type of rickets, renal phosphate regulation was assessed. Results showed low phosphate levels, low tubular reabsorption of phosphate (TRP) at 80% and normal vitamin D levels, and the patient was diagnosed with XLH-linked rickets. Treatment with phosphate 40 mg/kg/day and 1-alpha vitamin D was initiated. At 3 months follow-up, all values had normalized; however, phosphate levels were higher than normal. After a further 3 months, the child was referred to an endocrinologist as no improvements were seen in phosphate biochemistry or lower limb deformities.
Endocrinological assessment highlighted short stature and low weight, generalized hypotonia, sitting with support, wide wrists and fontanels, rachitic rosaries, and angulation of tibias and ankles without organomegaly or components of the cardiovascular system (CVS)/chest abnormalities. Repeated laboratory analysis showed low calcium levels, high phosphate and PTH levels with normal ALP. The patient was re-diagnosed with vitamin D deficient rickets type 1 (1-alpha hydroxylase deficiency) as a result of a frame-shift mutation in the CYP27B1 gene. Phosphate treatment was discontinued while the patient continued 1-alpha calcidiol only, which normalized all laboratory values and over time induced improvements of bone deformities. The patient has been stable for the past 2.5 years, highlighting that continued monitoring and re-evaluation of the underlying disease is required to adequately manage patients with mineral and bone abnormalities. When diagnosing a patient, it is vital to bear in mind that empirical use of 1-alpha calcitriol can mask 1-alpha hydroxylase deficiency and that in XLH-related rickets, very high ALP and PTH are unusual, although it can be difficult to normalize phosphate levels.
Case 3: Ahmed Yousef, UAE: Sheikh Khalifa Medical City, Abu Dhabi, UAE. A 16-month old boy presented to the clinic after unsuccessful treatment in various hospitals with systemic pain, especially under movement or when touched, leading to irritability, crying and failure to thrive. His symptoms started to become apparent at 10 months old and included arrested gross motor development, deformity in all extremities, respiratory distress and recurrent chest infections.
Initial biochemical analysis showed low levels of phosphate, calcium, and 25(OH)D, whereas ALP and PTH were at high levels. The patient was treated with 15 μg/day calcitriol and oral calcium carbonate up to 6400 mg/day.
A slight clinical improvement could be observed with stabilization of calcium levels; however, PTH and ALP remained high. Repeat analysis for 1,25 (OH)2D in another laboratory revealed a much higher level than previously reported. Genetic testing confirmed a pathological novel mutation in the VDR gene, which led to a diagnosis of vitamin D-dependent rickets type 2 (VDDR2) or hereditary vitamin D-resistant rickets.
Subsequently, the patient was treated with long-term continuous infusion of calcium to which he responded well. Radiological features improved, pain subsided, and the child achieved growth expected for his age. After a few years, the patient returned to the clinic with pain in his limbs, worsening gait and leg deformities. Home treatment with calcium carbonate 324 mg/ kg/day and cholecalciferol 10,000 IU daily was not effective, and laboratory values indicated a worsening of the condition with calcium, phosphorus and magnesium well below the normal range and ALP and PTH above normal levels. Vitamin D levels had improved to within the normal range and urine creatinine analysis did not show any abnormalities.
A repeat continuous infusion with calcium gluconate 570 mg/kg/24 hour, cholecalciferol 600,000 IU injection, hydrochlorothiazide 35 mg once daily (OD), magnesium oxide 200 mg twice daily (BID) and phosphate 250 mg BID was administered, which brought calcium, phosphate and PTH levels close to or within the normal range. This case highlights that, despite a correct diagnosis and correct initial management, it is essential to follow up with patients regularly and adjust their treatment to match the body’s needs during different growth phases.
Disorders of phosphate homeostasis
Physiology of phosphate homeostasis: Dieter Haffner, Germany: Medizinische Hochschule Hannover, Germany. Phosphates are pivotal for the regulation of metabolic processes and cellular functions. Phosphate is a constituent of DNA, membrane lipids, high-energy phosphates, and second messengers such as inositol trisphosphate, cyclic adenosine monophosphate and cyclic guanosine monophosphate and is used in protein phosphorylation. It is essential for the regulation of enzyme and receptor activities, energy metabolism, cell signaling, nucleic acid synthesis and membrane function, as well as skeletal health and integrity and growth. Physiology has evolved to conserve this rare mineral through efficient use of phosphate transport systems. Phosphate homeostasis is tightly regulated via feedback loops by hormones including the PTH and fibroblast growth factor 23 (FGF-23) and phosphorus is mainly contained within the intestine, kidney and bone.
The regulation of phosphorus is complex and involves both acute and chronic processes. Recent evidence showed that FGF23 regulates serum phosphate concentration and calcitriol metabolism. FGF23 is secreted in response to hyperphosphatemia and vitamin D decreasing renal phosphate reabsorption by lowering NPT2a and NPT2c expression and diminishing calcitriol (Vitamin D) synthesis by inhibiting 1α hydroxylase and stimulating its catabolizing enzyme 24, 25 hydroxylases. To achieve this, FGF23 binds to and activates a composite receptor formed by the conjunction of FGF receptor 1 (FGFR1), FGFR3 and or FGFR4 with klotho. Overexpression of FGF23 results in marked increase in urinary phosphate excretion and severe hypophosphatemia leading to many bone disorders.
Klotho is a transmembrane protein expressed on the surface of tissues like kidneys, parathyroid glands, brain and skeletal muscle, and acts as a co-factor that is mandatory for FGF23 activity. In the kidney, Klotho is mainly expressed in the distal tubule, whereas FGF23 exerts its action on the proximal tubule. The mechanism by which FGF23 modifies proximal tubule functions is unknown. Furthermore, a soluble form of Klotho provides a non-enzymatic molecular scaffold for FGF23 hormone signaling.
High dietary or serum phosphorus is reduced by stimulation of PTH or increased secretion of FGF23 and a feedback loop between both systems, which in turn reduce phosphorus reabsorption in the kidney. An additional mechanism acts via decreased vitamin D synthesis in the kidney as a result of high FGF23 secretion and a resulting reduction in intestinal phosphorus absorption. However, in XLH, an excess of FGF23 impairs renal phosphate and vitamin D metabolism. In patients with advanced or chronic kidney disease (CKD), elevated PTH levels are required to eliminate excess phosphate (a known cardiovascular toxin) and counterbalance vitamin D deficiency. At later stages of CKD, PTH levels progressively increase with declining glomerular filtration rate due to hyperphosphatemia, hypocalcemia and vitamin D deficiency, in order to increase phosphaturia, calcitriol synthesis and serum calcium.
Hypophosphatemic rickets: clinical features, genetics and differential diagnosis: Zulf Mughal, UK: Royal Manchester Children’s Hospital, UK. The clinical features of hypophosphataemic rickets often include impaired skeletal mineralization, impaired linear growth, impaired muscle function, propensity to dental abscess development, premature fusion of cranial sutures, fractures and pseudo-fractures, hearing impairment and calcification of spinal and paraspinal ligaments in adults, which results in stiffness.
A biochemical work-up including serum calcium, phosphate and ALP, as well as serum sodium, potassium, creatinine, 25OHD and 1,25(OH)2D should be performed. Plasma analysis should include PTH and intact FGF23. Urine testing should always be performed and analyzed for TRP, renal tubular maximum reabsorption rate of phosphate to glomerular filtration rate (TmP/ GFR), calcium/creatinine ratio, urine amino acids, urine potassium and bicarbonate, as well as urine protein/creatinine ratio and low-molecular-weight protein. A schematic developed to diagnose patients with low serum phosphate demonstrates the sequence of assessments necessary to identify the cause of hypophosphatemia (Figure 1).
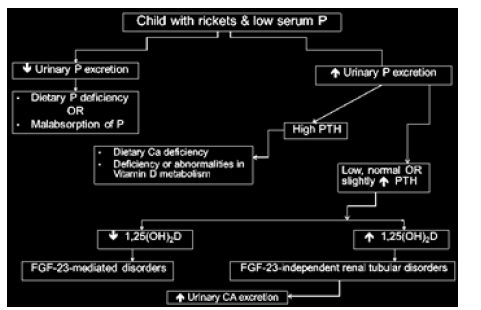 Figure 1:Decision chart for identification of the underlying cause of hypophosphatemia rickets. P, phosphate.
Figure 1:Decision chart for identification of the underlying cause of hypophosphatemia rickets. P, phosphate. Unusual Cause of Hypophosphataemic Rickets: Najlla Al- Jassas, KSA: King Fahd Specialist Hospital and Research Centre, Dammam (please check), KSA. The case of a 10-year-old Saudi boy with polyostotic fibrous dysplasia (FD) and hypophosphataemic rickets was reported. FD consists of rare and benign osseous lesions of unknown aetiology and represents 2.5% of all bone and 7% of benign bone tumors in young, predominantly male patients. Although hypophosphatasemia and hypophosphataemic rickets in patients with FD is infrequent, a renal tubulopathy including some degree of phosphate wasting is one of the most commonly associated extra-skeletal manifestations. It is now understood that FD-associated phosphate wasting arises from overproduction of FGF23 by abnormal osteogenic precursors. Treatment with bisphosphonate has been promising.
The patient presented at the age of 6 years with right thigh pain and limping for the past year. X-ray of the right femur revealed a lytic lesion and active rickets. In addition to a history of fractures, on physical examination, the boy appeared well, but showed symptoms of wide wrists and ankles, antalgic gait and heterogeneous increased density of the frontal skull region with extensive craniofacial involvement. The patient did not display signs of skin hyperpigmentation or endocrine hyperfunction. Bone biopsy confirmed the diagnosis of FD with a bone mineral density (BMD) Z-score of –3.6 SD below the mean.
Biochemical investigation revealed low phosphate, phosphaturia, normokalaemia, normal PTH and elevated FGF23 and ALP levels. Therefore, the diagnosis was extended to polyostotic FD with hypophosphataemic rickets due to vitamin D insufficiency. Genetic testing did not reveal mutations in the GNAS1 gene, the product of which is responsible for G-protein function and involved in hormonal signaling. The patient was treated for rickets with 1-alpha calcidol, oral calcium and oral phosphate and zoledronic acid infusion every 6 months for FD. Bisphosphonate was used due to its ability to reduce pain and fracture rate. Bisphosphonate has also been implicated in FD lesion size reduction and filling in of bony defects in adults and children; however, the effect is not consistent across patients. On follow-up, while progression of FD has been positively impacted with zoledronic acid in terms of lack of new lesions and improvement of BMD, the main challenge identified with this patient was persistent elevation of FGF23 and phosphaturia, which may be the result of non-adherence to rickets treatment. This case highlights the challenges of treating XLH in the presence of active FD lesions and the benefit of zoledronic acid in the management of pain and disease progression.
XLH with Mild Renal Phenotype: Bashir Elnaem, KSA: Madinah Maternity and Children Hospital, Madinah, KSA. Identifying the type of hypophosphataemic rickets requires detailed analysis. A boy of just under 6 years presented to gain a second opinion for his bowed legs. He showed symptoms of severe skeletal deformity including short stature, frontal posing and palpable sagittal suture, craniosynostosis, small dental abscess, bilateral genu, coxa Varus and mild renal phenotype without rickets rosary or organomegaly.
He has been treated with different vitamin D preparations for rickets by three individual hospitals for the past 2 years but has not received medication for the past 2 months. His medical history was unremarkable without neonatal or systemic problems and he showed no signs of developmental abnormalities. Initial investigations identified low phosphate levels (0.9 mmol/L), low TRP (80%) and low TmP/GFR (0.81 mmol), as well as radiological signs of rickets. All other laboratory values were within normal range. Both parents tested within the normal range for calcium, phosphate and ALP.
The child was treated initially with phosphate 40 mg/kg/day and 1 μg/day of one-alpha, which was subsequently increased to 125 mg/kg/day and was seen by an orthopedic surgeon for epiphysiodesis, a neurosurgeon and dentist. Over time (2 years) his phosphate levels normalized but remained close to the lower limit, while his PTH levels increased above normal. Calcium, ALP and TRP% remained stable and his bone deformities improved markedly without any signs of nephrocalcinosis. This patient case demonstrates the need for adequate assessment of potentially underlying comorbidities which may mask initial laboratory measurements and potentially result in inadequate treatment.
Challenges in Hypophosphataemic Rickets Management
Traditional and new management of XLH: Dieter Haffner, Germany: Medizinische Hochschule Hannover, Germany. XLH requires life-long management from a range of specialists owing to the multitude of symptoms and organ systems affected by this inherited disorder. Early diagnosis and management is vital to address symptoms early before they impact development and result in functional limitations and poor quality of life. Combination therapy with multiple doses of oral phosphorus and active vitamin D analogues to counter calcitriol deficiency, prevent secondary hyperparathyroidism and increase phosphorus reabsorption from the gut are the conventional treatment regimens. The overall treatment goals include healing rickets for clinical and radiological signs, control pain, encourage growth within the normal range and prevention of rickets in infants with a positive family history.
Despite the wide-spread use of these therapies, patient response is variable and the disease itself is not cured. Furthermore, there is an increased risk to develop side effects such as nephrocalcinosis and hyperparathyroidism, as well as stimulation of FGF23 secretion, which in turn promotes increased phosphate leakage. Strict monitoring every 3 months for biochemical values and every 12 months for ultrasound and radiological features is recommended. Before burosumab was available, conventional treatment was tailored to address specific clinical features and individualize patient management. However, especially in children and adolescents, inconsistent adherence to therapy might negate positive treatment outcomes. In addition to medicine-based therapy, collaboration with experienced orthopedic surgeons and physiotherapists are recommended to correct complex misalignments, reinforce muscles, improve joint stability and provide a good physical framework in which children can grow to their adult stature.
A novel fully human anti-FGF23 monoclonal antibody therapy, burosumab, has demonstrated effective inhibition of the FGF23- mediated pathway and phosphate excretion. It has been trialled as 2-weekly and 4-weekly injectable formulation in children and adults with XLH in Phase 2 and 3 studies, where it demonstrated significant improvements in the rickets severity score, radiographic features, walking test, growth velocity, standing height and markers of bone turnover to support fracture healing. Furthermore, normalisation of ALP, vitamin D and serum phosphate levels have been reported. In terms of patient-reported outcomes, burosumab significantly improved stiffness and physical functions, and provided alleviation from pain compared with placebo. These results led to burosumab approval by both the EMA and the FDA.
Genotype and Phenotype of XLH in Riyadh: A Case Series: Fahd Al-Juraibah, KSA: King Abdullah Specialized Children’s Hospital, Riyadh, KSA.Normal bone growth and mineralization require adequate calcium and phosphate. Hypophosphatemic rickets are a result of renal phosphate wasting due to primary renal tubular defects in phosphate reabsorption or the generation of excessive amounts of phophatonins (FGF23, MEPE, FRP4 and FGF7), which inhibit renal tubular reabsorption of phosphate. Causes of phosphopenia include non-hyperphosphaturic causes such as low body weight, vitamin D deficiency, total parenteral nutrition, short bowel syndrome, chronic diarrhea, and aluminum/ calcium-containing antacids, as well as hyperphosphaturic pathways including non-FGF23-mediated, FGF23-mediated and those governed by high PTH.
A case series of six patients with XLH in Riyadh was discussed highlighting the different symptoms patients can present with and the importance of assessing their laboratory values considering these symptoms. All patients, evenly mixed by gender, presented very early on in life between the ages of 5 months and 1 year often with tell-tale deformities. In all but one of the patients, the family history of rickets was known, supporting diagnosis by genetic testing. Heterozygous mutations in the PHEX gene, the product is thought to be involved in bone and dentin mineralization and renal phosphate reabsorption, were the most prominent causes of XLH, albeit different mutations within the same gene were causing the disease (c.2070+5G>A and c.1682G>A). A homozygous mutation in the DMP1 gene exon 2, a protein critical for correct mineralization of bone and dentin, as well as a novel frameshift mutation of the PHEX gene (c.1077del) have been identified as contributing to a hypophosphataemic rickets phenotype.
New Management of XLH: KSA Experience: Mohamed Al- Dubayee, KSA: King Abdullah Specialized Children’s Hospital, Riyadh, KSA. In addition to conventional treatment of XLH, a novel fully human anti-FGF23 monoclonal antibody therapy, burosumab, has demonstrated effective inhibition of the FGF23-mediated pathway, thus stimulating renal phosphate reabsorption and increasing serum phosphorus and active vitamin D levels.
Monoclonal antibodies are an effective therapy characterized by high target specificity. The FDA and EMA have recently approved burosumab for the treatment of XLH in adult and pediatric patients 1 year of age and older for the treatment of XLH with radiographic evidence of bone disease in children 1 year of age and older and adolescents with growing skeletons, respectively.
In children, burosumab demonstrated improvements in serum phosphorus levels, renal tubular phosphate reabsorption, serum 1,25(OH)2D and ALP into the normal range with both 2-weekly and 4-weekly administrations and a rapid onset of action within the first 2–4 weeks. When compared with conventional therapy of oral phosphate and active vitamin D, burosumab showed superiority leading to the adoption of this treatment by the Saudi Food and Drug Authority.
The recommended starting dose is 0.8 mg/kg, rounded to the nearest 10 mg administered every 2 weeks in children and 1 mg/ kg rounded to the nearest 10 mg up to a maximum dose of 90 mg every 4 weeks in adults. The first dose of burosumab should be given in an inpatient setting to allow observation of any side effects. Oral phosphate and vitamin D analogues must be stopped 1 week prior to burosumab initiation to allow fasting serum phosphate to drop below the reference range for age prior to initiation of treatment. Furthermore, burosumab should not be initiated if serum phosphorus is within or above the normal range for age or in patients with renal impairment or end-stage renal disease.
Prior to initiation of subcutaneous burosumab injection, fasting baseline tests should be performed to assess standard biochemistry of the patient. Testing should include serum complete blood count, urea and electrolytes, bone profile, liver profile, ALP, PTH, 25(OH)D, 1,25(OH)2D, urine phosphate, calcium and creatinine to calculate TmP/GFR, as well left-hand X-ray for a rickets survey. After starting burosumab, fasting serum phosphate should be monitored every 2 weeks for the first month of treatment, every 4 weeks for the following 2 months and thereafter every 3 months or as appropriate. Serum phosphorus should be maintained between 1–1.6 mmol/L. The burosumab dose may be increased stepwise up to approximately 2 mg/kg if serum phosphorus is below the reference for age; however, dosing should not be adjusted more frequently than every 4 weeks and adequate monitoring should be provided.
Burosumab is the first treatment to target regulatory pathways instead of achieving clinically normal levels of minerals by dietary supplementation.
Skeletal Disorders Beyond Rickets
An approach to a child with multiple fracture: Rasha Hamza, Egypt: Ain Shams University, Cairo, Egypt. Recurrent fractures in children are not coincidental and the underlying disease may present at differing severity across a wide spectrum, creating a challenge for diagnosis. Patients may present with several numbers of fractures, stature abnormalities, hearing problems, dental or eye abnormalities, deformities, osteopenia, bone formation abnormalities and random calcifications.
Bone strength is affected when there is an imbalance of bone formation and bone resorption, a finely tuned system that is regulated via the receptor activator of nuclear factorkappa B-receptor activator of nuclear factor-kappa B ligandosteoprotegerin (RANK-RANKL-OPG) pathway. RANKL/RANK signaling regulates osteoclast formation, activation and survival in normal bone modelling and remodeling and in a variety of pathologic conditions characterized by increased bone turnover. OPG protects bone from excessive resorption by binding to RANKL and preventing it from binding to RANK. Thus, the relative concentration of RANKL and OPG in bone is a major determinant of bone mass and strength. Bone strength is further determined by bone mineral density and material as well as structural properties such as geometry, microarchitecture, mineralization, collagen and non-collagen proteins. Bone mass, however, can be governed by hormonal factors such as growth hormone and nutritional factors including vitamin D and calcium among others. There are currently no guidelines for the management of fractures in healthy children, and general measures to improve bone health include weightbearing physical activities, diet adequate in calcium and correction of vitamin D deficiency.
In a child with a history of fractures (vertebral or long bones), bone health as well as dietary calcium intake need to be assessed. Furthermore, clinical/social and laboratory assessments of serum calcium, serum phosphorus, serum ALP, serum 25OHD and serum PTH together with radiological imaging and genetic testing should be performed prior to a bone biopsy for ultimate confirmation for disease. Various antibody-based therapies have been developed to regulate imbalanced bone development pathways.
Challenges in the Management of Hypocalcemia: Amir Babiker, KSA: King Abdullah Specialized Children’s Hospital, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs, Riyadh, KSA. Calcium is a physiologically important element governing many functions throughout the body. Calcium plays a part in neuromuscular excitability, blood coagulation, hormonal secretion and enzymatic regulation, as well as providing structural integrity to the skeleton. Calcium homeostasis is regulated by the hormones PTH and 1,25(OH)2D (the active form of vitamin D). Hypocalcemia stimulates PTH secretion, which acts via three mechanisms to increase calcium levels: firstly, PTH stimulates the release of Ca from the bone, in part by stimulating bone resorption, secondly PTH decreases urinary loss of Ca by stimulating Ca reabsorption and lastly, PTH indirectly stimulates Ca absorption in the small intestine by stimulating synthesis of 1,25(OH)2D in the kidney.
Causes of hypocalcemia tend to fall within three categories: hypo parathyroid, non-Para hypothyroid or PTH resistance with each category containing multiple diseases itself. Clinical features include neuromuscular irritability, neurological signs and symptoms, abnormal mental status, ectodermal changes, smooth-muscle involvement, ophthalmological manifestations and cardiac features. Extensive laboratory investigations should be performed to identify the exact cause of calcium deficiency and treatment should be based on the underlying cause. If the patient has a vitamin D imbalance, vitamin D and calcium supplementation should be prescribed, whereas acid citrate dextrose and calcium or PTH replacement therapy should be employed if PTH imbalance is diagnosed.
An Approach to the Child with Hypercalcemia: Sarah Ehtisham, UAE: Mediclinic City Hospital, Dubai Healthcare City, Dubai, UAE. Hypercalcemia is a disorder which is specified by excessive calcium levels in the blood serum. Clinical symptoms include insidious onset, a general feeling of discomfort, behavioral change, constipation, anorexia, weight loss, dehydration, polyuria and polydipsia, bone pain, hypertension and short QTc.
Hypercalcemia can have many underlying causes that are stratified based on PTH level. Low PTH can have genetic causes or secondary causes such as malignancy, vitamin D excess, adrenal insufficiency or thyrotoxicosis. Normal PTH levels in hypercalcemia can be associated with familial hypocalciuric hypercalcemia’s and elevated PTH can be attributed to hyperparathyroidism or parathyroid carcinoma. Key investigations that should be performed to identify the underlying cause of hypercalcemia include bone profiling (calcium, phosphate, ALP and AIb), renal function assessment (electrolytes and creatinine), PTH, 25(OH) D, urine calcium/creatinine ratio or 24 hour urine calcium, store serum (may require 1,25(OH)2D, PTH-related peptide or genetic analysis) and potentially renal ultrasound, parental bloods, skeletal survey and parathyroid imaging.
The treatment of hypercalcemia includes the lowering of calcium at first, but also correcting the underlying disease. Calcium-intake reduction, promotion of mobility, as well as an increase in urinary calcium excretion through hydration and diuretic use support the reduction of overall systemic calcium. In addition, a reduction in PTH secretion, intestinal calcium absorption and bone resorption and treatment with cinacalcet, glucocorticoids, bisphosphonates, calcitonin, dialysis or parathyroidectomy contribute to a calciumlowering effect.
Genetic Backgrounds of Bone Diseases in the UAE: Asma Deeb, UAE: Mafraq Hospital, Abu Dhabi, UAE. Bone diseases are often associated with dysregulation of complex metabolic or hormonal systems and constitute an interesting spectrum in pediatrics. Genetic testing may be required to confirm diagnosis of bone disease if initial treatment is ineffective or no clear diagnosis can be drawn from physical, biochemical and radiological assessments, especially in a region with a high rate of consanguinity. As genetic testing is not widely available in the Middle East, regional and international collaborations are crucial to support this line of investigation. A series of cases in whom genetic diagnosis was made was presented highlighting the complexity and multiple pathways involved in bone disease.
In a family with consanguineous parents, seven of their 11 children presented with symptoms of hematuria, loin pain or recurrent urinary tract infection. Although symptoms were relatively consistent between the siblings, calcium, magnesium, hypercalciuria and nephrocalcinosis status were relatively varied. Genetic analysis identified a novel mutation in the CLDN16 gene as responsible for this phenotype. In another case, heterozygous mutation in the CaSR gene (Ser113Cys) led to the diagnosis of autosomal dominant hypocalcemia and familial hypocalcemia hypocalciuria. Two partial gene deletions have also been associated with syndromes of abnormal calcaemia, namely William syndrome and DiGeorge syndrome.
A novel FOXI1 homozygous missense mutation, p.L146F, located within evolutionary highly conserved residues of the FOXI1 protein, was the underlying cause of patients presenting with earlyonset sensorineural deafness and distal renal tubular acidosis based on dysfunction of electrolyte regulation. Fanconi syndrome has been identified in a patient with mutations in the SLC34A1 gene on chromosome 5q35 resulting in poor growth, subtle dysmorphic features and low phosphate. In another case, a 5-year-old child presented with increasing deformity, hypophosphatemia, high 1,25(OH)2D and normal FGF23 levels. Genetic analysis did not identify abnormalities in the PHEX or SLC34A3 genes, excluding XLH and hereditary hypophosphataemic rickets with hypercalciuria from the diagnosis, respectively. Potential mutations in the FGF23 gene could be responsible for this phenotype.
Mutations leading to abnormal bone growth and abnormal growth plates include COL1A gene mutation type 3, LIFR (5p13.1) gene mutation leading to Stuve-Weidemann syndrome/Schwartz- Jampel type 2 syndrome, MMP2 gene mutation (TPOp.R665Q, c.1994G>A) leading to multicentric osteolysis and nodulus arthropathy, homozygous ADAMTSL2 gene mutation (c.938T˃C, p.M313T, exon 10), homozygous mutation of the EVC gene (c.1405delC) leading to Ellis-van Creveld syndrome, GALNS gene mutation, FGFR3 gene mutation, deletions in the CHRNA1 gene associated with congenital myasthenia, heterozygous mutations in the NPR2 gene causing short stature, COL2A1 gene mutation leading to sporadic Spondyloepiphysia congenita, homozygous RAB33B gene mutation causing Smith McCort dysplasia and a novel homozygous mutation in the PAPSS2 gene (c.826_828delGAG(p. E276del)) leading to brachyolmia type 4 with mild epiphysial and metaphyseal changes.
Meeting discussions
Lively discussions around all presented topics dominated the day. Table 1 captures the most discussed topics by theme Figure 2.
Figure 2: ASPED steering committee with regional and international speakers.
Table 1: Discussion themes throughout the meeting.
EMA, European Medicines Agency; FDA, Food and Drug
Administration; FGF23, fibroblast growth factor 23; GCC, Gulf
Cooperation Council; GFR, glomerular filtration rate; IGF-1, insulinlike
growth factor-1; KSA, Kingdom of Saudi Arabia; MRI, magnetic
resonance imaging; PTH, parathyroid hormone; RAAS, reninangiotensin-
aldosterone system; RSS, rickets severity scoring; XLH,
X-linked hypophosphataemia
To view fulltext of article:https://biomedgrid.com/fulltext/volume3/arab-society-for-pediatric-endocrinology-and-diabetes-asped-masterclass-in-pediatric-bone-disease.000678.php

Comments
Post a Comment